Cross-Linked HA Biomaterials
HA presents many inherent advantages as a foundation for biomaterials. In addition to derivatization, cross-linking is another means of engineering HA’s physicochemical properties. Depending on the cross-linking molecule and reaction chemistry, a wide variety of HA materials can be created, ranging from films with relatively low water content to highly swelling hydrogels. Most HA cross-linking methods fall into either of two general schemes: a one-step procedure consisting of the exposure of HA to a cross-linker, or a two-step procedure in which a highly reactive HA derivative is first synthesized and then cross-linked in a subsequent reaction. Following is a brief listing of commonHA cross-linking techniques.
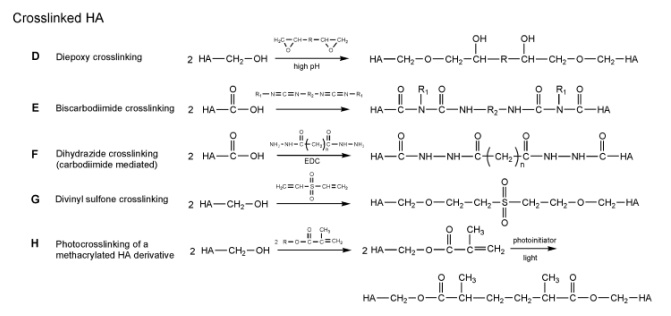
Diepoxy cross-linking
Almost 60 years ago, Laurent and coworkers created diepoxy-cross-linked HA hydrogels using polyethylene glycol diglycidyl ether.Others have extended the utility of the diepoxy chemistry to other cross-linkers, including ethylene glycol diglycidylether (a bifunctional crosslinker) and polyglycerol polyglycidylether (a trifunctional cross-linker). Interestingly, detailed studies of this chemistry have shown that at low pH, diepoxy compounds form ester linkages between carboxyl groups, while at high pH, they form ether linkages between hydroxyl groups. Utilizing this fact, diepoxyoctane has been used to cross-link HA in a two-step double-crosslinking
treatment: HA is reacted with diepoxyoctane at low pH, forming ester cross-links between HA chains, and then a second diepoxyoctane reaction is subsequently carried out at high pH, forming ether cross-links.
Carbodiimide-mediated cross-linking
Carbodiimide-mediated reactions are commonly used for reactions between carboxylic acids and amines. These reactions, however, are highly sensitive
to pH and readily form an unreactive acylurea moiety when applied to HA reactions; therefore, the applications for which carbodiimides can be used with HA are somewhat limited. Fortunately, several researchers have found specific conditions under which carbodiimide-mediated reactions can allow HA cross-linking.The simplest of these methods uses solely carbodiimide to induce inter- and intramolecular cross-links on HA;in other words, these materials can be synthesized without the use of exogenous cross-linkers. To form cross-links, the carbodiimide-mediated reaction is performed on films of at least 70 weight-percent HA in acetone- or ethanol–water solutions. Similar reactions carried out with HA in solution did not yield cross-linked materials, perhaps due to the formation of unreactive acylureas. Challenged by this tendency of carbodiimide-mediated reactions to form unreactive acylureas on HA, Kuo, et al. created a biscarbodiimide cross-linking technique.
Several biscarbodiimides were synthesized and covalently bound to HA through a mechanism similar to the carbodiimide-mediated creation of an acylurea, producing
aromatic or aliphatic cross-links between HA molecules. Finally, several efforts have utilized carbodiimides to mediate reactions between HA’s carboxyl groups and the
amines of bifunctional cross-linkers. Bullpitt and Aeschlimann made use of reactions mediated with carbodiimide and 1-hydroxybenzotriazole (HOBt) to couple activated amines (pKa
Aldehyde cross-linking
Formaldehyde and glutaraldehyde have long been used to cross-link proteins for tissue preservation. Formaldehyde is used to create Biomatrix’s Hylan-A, a cross-linked form of HA that is water-soluble, but more viscous and elastic than native HA. Glutaraldehyde has been used to crosslink HA to yield materials with high resistance toward degradation. Tomihata and Ikade used glutaraldehyde in acidic acetone–water solutions to create cross-linked HA films of low water content. Hu et al. compared
glutaraldehyde and carbodiimide-mediated reactions and found that glutaraldehyde yielded more highly crosslinked materials suitable for use as three-dimensional
tissue scaffolds.
Divinyl sulfone cross-linking
Insoluble HA hydrogels can be formed by cross-linking HA with divinyl sulfone , yielding materials such as Biomatrix’s Hylan-B gel. At high pH, divinyl sulfone creates sulphonyl-bis-ethyl crosslinks between HA’s hydroxyl groups. Depending on the reaction conditions, materials ranging from soft gels to firm solids
(e.g., membranes and tubes) can be formed. Compared to native HA, Hylan-B has an extended residence time in vivo, particularly in sites where low mechanical force is
imposed on the implant.
Photocross-linking
While the cross-linking methods discussed above yield a wide variety of stabilized materials, most of these techniques proceed under physiologically incompatible conditions. Furthermore, many of these methods crosslink immediately upon mixing of the HA with the crosslinker. Because HA forms highly viscous solutions, it is often difficult to obtain homogenous materials in this manner. Photocross-linking, however, is a method that reacts only upon exposure to the appropriate wavelength
of light; therefore, the reactants can be thoroughly mixed before cross-linking is initiated. Furthermore, photocross-linking methods are capable of proceeding under
physiological conditions without detrimental effects to copolymerized bioactive molecules or encapsulated cells.To this end, HA has been modified with several photocross-linkable groups, including cinnamoyl, coumarin, and thymine;methacrylic anhydride; glycidyl methacrylate; and styrene. Additionally, sulfated HA has been modified with 4-azidoaniline, yielding an HA derivative suitable for patterning features as small as 100 mm, which may be useful for directing cell growth or adhesion in new biomaterials.