HA Derivatives
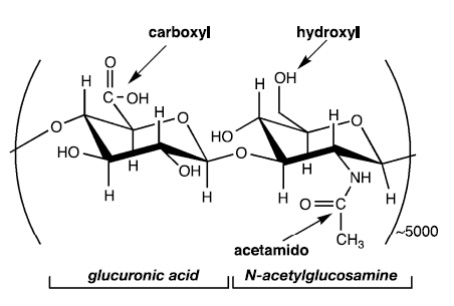
Many studies focus on three main types of HA derivatization strategies: esterification, carbodiimide-mediated modification, and sulfation. These simple modification strategies can be used to create HA derivatives that are suitable for direct application or for subsequent crosslinking procedures.
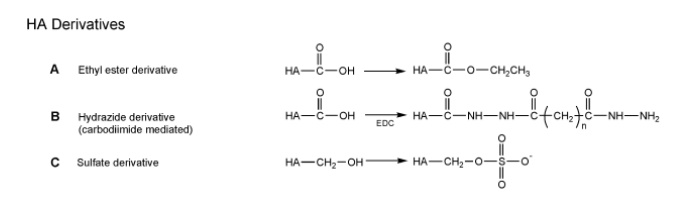
Ester derivatives
To create more hydrophobic forms of HA that have added rigidity and are less susceptible to enzymatic degradation, researchers have esterified HA’s carboxyl
groups.The modification is carried out through an alkylation step of HA with an alkyl halide, yielding derivatives with 0–100% modifications of the available carboxyl groups. As the percent of HA esterification increases, these materials become more rigid and more hydrophobic. An increased hydrophobic nature means that the HA derivatives are less soluble in water and less susceptible to enzymatic degradation. Fidia Advanced Biopolymers have created a range of HA esters, including
ethyl esters and benzyl esters of HA (Hyaff-11). Through a variety of processing steps, these HA esters can be shaped into fibers, membranes,sponges, and microspheres.
Carbodiimide-mediated derivatives
Reactions mediated by carbodiimides (e.g., 1-ethyl-3-(3’-dimethylaminopropyl) carbodiimide or EDC) are a common means of covalently binding the carboxyl group of one bioactive molecule with an amine of another (e.g., reactions between amino acids during peptide synthesis).However, carbodiimide reactions are very sensitive to pH and often result in the formation of an unreactive intermediate, acylurea, from carboxyl groups. Therefore, depending on the reaction conditions, carbodiimidemediated reactions on HA can result in very low coupling yields.
Pouyani and Prestwich gained a detailed understanding of these reactions, and as a result, chose hydrazide derivatization as a means of increasing
the utility of carbodiimide in HA modifications. Prestwich and coworkers have continued to develop a wide variety of HA-hydrazide derivatives for use as
drug delivery devices, cellular probes, and HA-protein conjugates.
Sulfated derivatives
To create a blood-compatible molecule that mimics heparin, researchers have modified HA with sulfate groups. The sulfation is completed through a reaction of sulfur trioxide pyridine with HA’s hydroxyl groups, yielding a range of sulfation from one to four sulfur groups incorporated per HA disaccharide. This sulfation modification creates HA derivatives that are structurally and chemically similar to heparin; thus these materials are studied in cardiovascular applications.